![]()
Search
|
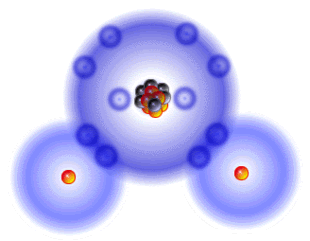
It's Sunday, my day for cutting myself some slack, but in the week of sparse communication, quite a bit has actually taken place. First of all, we are very close to posting the first page of the Japanese language section of zerorads.com! Secondly, we are also working on some material on bioremediation using plants. Thirdly, we hope to have some superb products in transit within the next days. My role has been to maintain a focus and try to move from the overview to the particular steps that might make a difference in many lives. It is not so easy for me to move from the big picture to the details and thus the slowness on my end. I am trying really hard to keep the material simple without watering it down to the point of igniting the indignation of experts. It is important to be reader-friendly so as to involve as many people as possible. We are each able to be proactive and this is ultimately what is going to affect the outcome for people, plants, pets, wild life, and ultimately Lady Gaia Herself. Caveats aside, in this post, I want to focus on the level of the smallest atoms and this evening (hopefully) the emphasis will go to how the radiation that is being released from Fukushima might impact this microscopic level. This will be followed by posts on what you can do to protect your health and garden. Atoms Every atom has a nucleus and neutrons as well as positively charged protons and orbiting electrons that are equal in number to the protons. The orbits are very specific. Only two electrons can occupy the innermost orbit or shell. There can be eight electrons in the next orbit. Reactivity depends on the outermost shell. If it is full, the atom is stable and hence more or less inert. If it has room for more electrons, it is reactive. This means it can form relationships and create molecules through relationship. Electrons form bonds through chemical reactions in which the outer orbits of the atoms share electrons with one or more partners. Water comprises roughly two-thirds of our physical bodies and two-thirds of the Earth. Since it is so abundant, let's use water as an example . The oxygen atom has 8 protons and therefore 8 electrons, but two of those electrons are in the inner shell/orbit and the remaining six fail to fill up the outer orbit which, as noted, has room for 8 electrons. This instability is resolved by partnering with two hydrogen atoms that agree to share electrons so each behaves as if the outer shell is complete. This bliss is called a covalent bond. Water Molecule
|
|||
For reasons that will hopefully become clearer and clearer, this is the best example we could use since water is not just the most pervasive chemical on our Planet, but it is being used to limit the meltdown that is occurring in the Fukushima reactors. Let's however continue. Cells All cells are composed of molecules — and molecules, as we have seen, are composed of atoms that are held together by chemical bonds. These bonds may be quite stable or more precarious. Breaking a stable bond requires more energy than breaking a weak bond. If the bond is broken, the molecule itself is transformed. Besides radiation, there are other "agents" that can cause a bond to break and thus contribute to free radicals: heat, including smoking; chemicals or chemical compounds such as herbicides and vapors; atmospheric pressure; toxins, including metals such as mercury; and various frequencies, including sound waves. This is why multiple exposures to various risk factors tends to worsen the prognosis for those exposed to radiation. The tissue damage caused by radiation and other factors is due to the breaking of chemical bonds in a manner that impairs function and therefore the integrity of the system in which the cell is a part. Cells are specialized and have to have the right design in order to carry out their roles. When the chemical unions are broken, the ensuing cellular degradation is marked by increased instability which tends to be unending. Some regard the cascade as a chain reaction, but I prefer to see it as a crescendo in which it becomes increasingly difficult to predict the ability of the body to recover from the ravages of electron chaos. To heal means to regain control over the chemical processes that are necessary for coherence, i.e., maintaining the integrity of the whole. Free Radicals For the last 50-60 years, there have been schools of thought in which all aging and degeneration is attributed to the maverick behavior of the free radicals that are created when the chemical bonds between electrons are broken. In health circles, the antidote for free radicals is an antioxidant, usually substances high in vitamins A, C, and E, but as I have been saying for years now, it is entirely possible that the properties attributed to vitamins are actually due to flavonoids, not vitamins, so the conclusions of many studies are difficult to interpret. However, what is unique about free radical scavengers is their ability to remain stable with or without the electrons pirated by the free radicals. What is most important is that the chain reaction is understood. When a stable union is broken by a frantic radical, the remnants of that union become radicals. The patterns of these radicals are also destructive and ultimately the damage caused undermines the quality of life and eventually survival itself. In theory, however, all we need to stop the cascading consequences are a couple of spare electrons. Just how difficult is this? Well it can be quite difficult or perhaps not so difficult, depending on many factors. I think this subject is complex enough that we can pause here and save radiation and scavengers for tonight and next week. Many blessings, Ingrid Naiman
Copyright by Ingrid Naiman 2011 |
|||
*The material provided on this site is for informational purposes only. The site owner is not a medical doctor. Information provided is not intended to replace the services of health care professionals. The content and products discussed have not been evaluated by the Food and Drug Administration. The information on this site and the products discussed are not intended to diagnose, treat, cure, or prevent any disease.